For years we have known about genetics which is the study of genes. We have also known about proteomics which is the study of proteins. These fields are extremely complicated to understand but incredibly easy to study in the sense that DNA and protein sequences can (and have been for many years) be synthesized because of molecule association.
Let me explain. DNA has three major components:
- Deoxyribose (a 5-carbon sugar; D in DNA)
- Phosphate (creates bond between deoxyribose molecues to form backbone)
- Nucleic Acids (A, C, G, T; NA in DNA)
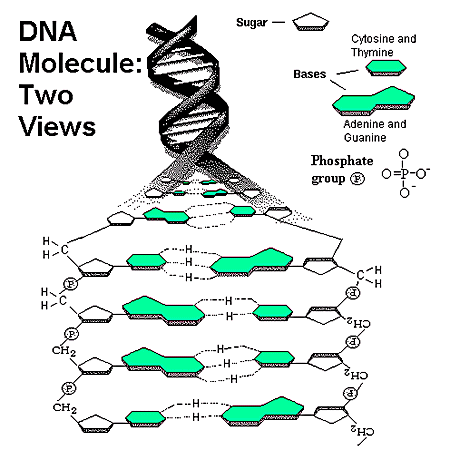
Pay close attention and you will notices that a big nucleotide (purine) always binds to a small nucleotide (pyrimidine). The specificity goes into even more exactitude! Not only can you predict that an A and G will always bind to a C or T, but you can predict that an A will ALWAYS bind to a T and a G will ALWAYS bind to a G. That's just the way it is. In DNA, no other nucleotides are present and other combination exists. Geneticists can fairly easily (although concept or procedure might be complex for non-biologists).
The same predictability is true in the study of proteins (proteomics). An amino acid has two end points which can attach to other amino acids to form (not fold) an oligopeptide, a true peptide, or a protein. Here is a list of the 20 amino acids in the human body:

Do you see the H3N+ and COO- at the top of each of the diagrams? The amino group (H3N+) from one amino acid will ALWAYS bind to the carboxylic acid group (COO-) of another. The only exception is when folding, other interactions can occur between sides groups but the protein backbone (line of H3N+ to COO-) linkages between amino acids) is not modified during fold/unfolding. Again, having a predictable pattern for linkage has facilitated the creation of proteins in labs for research purposes.
Sugar on the other hand has no such predictability... Or at least it didn't. Yesterday, ScienceDaily reported that scientists from the University of Georgia had found a way to synthesize complex carbohydrate structures in a much faster, more accurate process than ever before.
Why is this important?
Direct quote from article:
"The emerging field of glycomics has been severely hampered by a lack of robust, well-defined libraries of carbohydrate molecules, which are greatly needed to decipher the 'carbohydrate codes' used by cells for processes such as cell signaling, embryogenesis and neuronal development," said Pamela Marino, director of the glycobiology portfolio at the NIH's National Institute of General Medical Sciences.
This article linked to a similar article (March 23, 2009) in which German scientists boasted of having created "a device that builds these intricate [sugar] molecules in a few hours — rather than the months or years required with existing technology." This second article talks about the direct links to possible vaccines and medications that can spawn from this advancement.
Article [ScienceDaily]: New Method for Producing 'Libraries' of Important Carbohydrate Molecules
Article [ScienceDaily]: First Automated Carbohydrate 'Assembly Line' Opens Door To New Field Of Medicine
It's time you look at sugar a different way. Not all sugar is sucrose (table sugar) or glucose (fuel source and responsible for insulin secretion). No... Instead, glycobiology has been referred to by many prominent doctors as the latest frontier in medical and scientific research. To learn more about it, visit our site. Glyconutrients are eight essential sugars commonly found on surfaces of cells in the human body.
No comments:
Post a Comment